Background
Hypercalcemia (HC) is a frequent complication of multiple myeloma (MM) occurring in 20-30% of patients. This is often associated with renal dysfunction and both features are important myeloma defining events resulting in significant morbidity and mortality. Denosumab, a fully human monoclonal antibody that inhibits RANKL, has been evaluated in the prevention of skeletal related events in patients with newly diagnosed MM, as well as the treatment of bisphosphonate-refractory HC of malignancy (HCM). Cases of denosumab for HCM in MM patients with renal dysfunction have been described. Both denosumab and IV bisphosphonates (IVB) represent treatment options for HC in MM. We describe a comparison of patients with MM with HC who received denosumab vs IVBs.
Methods
We retrospectively identified patients age ≥18 with a diagnosis of MM with HC (corrected serum calcium level [CSC] >10.5 mg/dL). Patients were included if they received either denosumab or IVB (zoledronic acid [ZA] or pamidronate), between April 2016 and June 2020. The primary endpoint was complete response (CR), defined as normalization of CSC to less than 10.5 mg/dL. Secondary endpoints included HC relapse (CSC >10.5 mg/dL) and safety. Hypocalcemia was graded per CTCAE v5. Acute kidney injury (AKI) was defined using KGIDO criteria. Patients were followed-up for 56 days. Bivariate analyses were performed.
Results
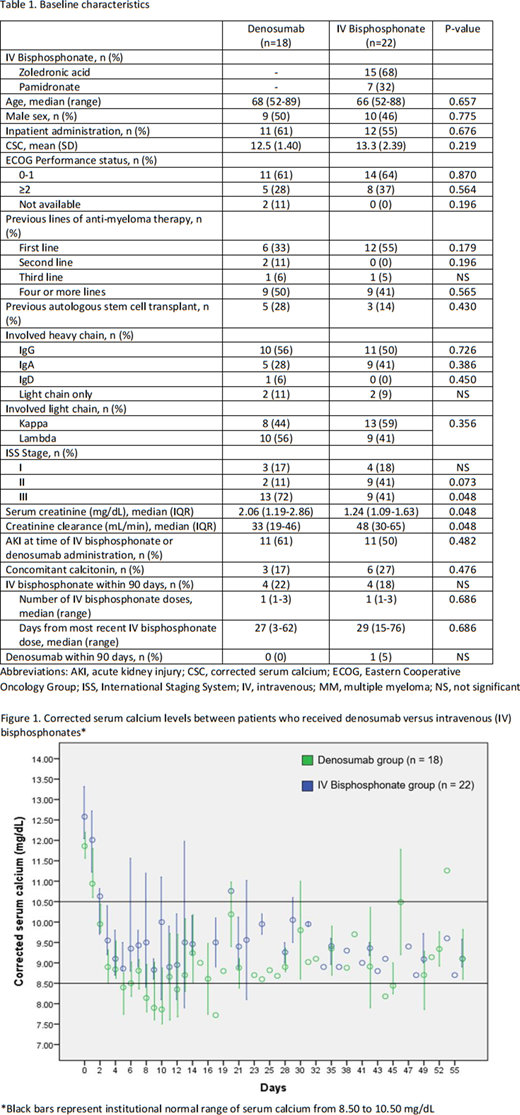
A total of 40 patients were included with 18 in the denosumab group and 22 in the IVB group, of whom 15 (68%) received ZA and 7 (32%) received pamidronate. Baseline characteristics are described in Table 1. Patients with newly diagnosed MM composed 33% and 55% of the denosumab and IVB groups, respectively. All patients in the denosumab group received 120 mg except one who received 60 mg, while in the IVB group, dose reductions occurred in 5/15 patients who received ZA (median dose, 4 mg; range, 3.3-4) and 4/7 patients who received pamidronate (median dose, 60 mg; range, 30-90). Most patients received HC treatment as an inpatient (58% inpatient vs. 42% outpatient). A minority of patients had received IVBs in the past 90 days. The mean CSC was 12.5 mg/dL (standard deviation [SD], 1.40) and 13.3 mg/dL (SD, 2.39) in the denosumab and IVB groups, respectively. Baseline serum creatinine (SCr) was higher and creatinine clearance (CrCl) was lower in the denosumab group (median SCr, 2.06 vs. 1.24 mg/dL, p=0.048; median CrCl, 33 vs. 48 mL/min, p=0.048). The CR rate by day 3-4 was 92% and 94% in the denosumab and IVB groups, respectively (p=NS). HC relapse occurred in 2 (12%) and 6 (29%) patients in the denosumab and IVB groups, respectively (p=0.257). Incidence of grade 1 hypocalcemia was similar between groups; however, incidence of grade ≥2 hypocalcemia was higher in the denosumab group. Incidence of new AKI was 28% (5/18) in the denosumab group 23% (5/22) in the IVB group (p=0.71). No patients in the denosumab group received an additional dose of denosumab within 14 days of initial dose. Three patients in the IVB group received an additional dose of an IVB within 14 days of initial dose. One patient, who was in the denosumab group, had refractory hypercalcemia and had not achieved CR at day 56.
Conclusions
We describe our experience with denosumab and IVB for the management of HC in patients with MM. The CR rate at 3-4 days was similar with either agent in our MM only population that was not bisphosphonate refractory. A higher incidence of grade 2 hypocalcemia was noted in the denosumab group. Conclusions on renal safety are limited by the small sample size and that patients in the denosumab group had a higher SCr on presentation. Denosumab and IVB represent acceptable agents for the management of HC in MM patients with further investigation necessary in those with renal dysfunction.
Lei:Fresenius Kabi USA: Consultancy; Trapelo Health: Consultancy; Bluebird Bio: Current equity holder in publicly-traded company; Bristol Myers Squibb: Current equity holder in publicly-traded company; Clovis Oncology: Current equity holder in publicly-traded company; Blueprint Medicines: Divested equity in a private or publicly-traded company in the past 24 months. Lou:Fresenius Kabi USA: Consultancy. Raje:Bluebird, Bio: Consultancy, Research Funding; Takeda: Consultancy; Immuneel: Membership on an entity's Board of Directors or advisory committees; Caribou: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; BMS: Consultancy; Celgene: Consultancy; Janssen: Consultancy; Karyopharm: Consultancy; Astrazeneca: Consultancy. Yee:Karyopharm: Consultancy; Oncopeptides: Consultancy; Sanofi: Consultancy; Takeda: Consultancy, Research Funding; Janssen: Consultancy; BMS: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Amgen: Consultancy, Research Funding; Celgene: Consultancy, Research Funding.
Denosumab is indicated for the treatment of hypercalcemia of malignancy refractory to bisphosphonate therapy. We describe the use of denosumab for hypercalcemia of malignancy in a multiple myeloma only patient population that is not bisphosphonate refractory. The use of denosumab for these patients was part of normal clinical practice in adherence to institutional policies and guidelines.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal